43 fda health claims on food labels
FDA Food Labeling Enforcement - LabelCalc Often, food manufacturers tell me that the FDA nutrition labeling rules seem very particular (some even say nitpicky). From label layouts to rounding rules and everything in between, I'll admit the FDA does have some pretty stringent guidelines for proper nutrition labeling. This is, however, for good reason. Legal Guide to Health Claims on Food | Law@Dayton The Nutrition Labeling and Education Act, which amended the FD&C Act in 1990, requires most foods to be labeled with serving sizes and specific nutrition information, and it sets standards for food labels that make certain health claims. The Fair Packaging and Labeling Act of 1966 spells out packaging requirements for food and other packaged goods.
Food Label Claims - Agriculture - Michigan State University Claims defined by the FDA and USDA. Ensure fair competition among producers or manufacturers. Provide basic information for consumers to reduce health and safety risks. Health claims reviewed by FDA are allowed to show that a food or food component may reduce the risk of disease or health related condition.

Fda health claims on food labels
PDF "GMO-Free" Claims and False and Misleading Food Labels—Why ... Through its butterfly label and marketing materials, the Non-GMO Project makes misleading and inaccurate claims, resulting in misbranding. It makes food safety claims that are false and misleading. It's claims interfere with consumers' ability to make wise food purchase decisions. Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,... Label Claims for Conventional Foods and Dietary ... there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990...
Fda health claims on food labels. Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified.". Authorized Health Claims: Claims that have significant scientific agreement (SSA). Factual Food Labels: Health Claims - University of Texas ... According to the United States Food and Drug Administration (FDA) there are only three categories of claims that are approved to be printed on food packaging: health claims, nutrient claims, and function claims. Generally, these labels are found on the front side of the food package in emphasized lettering. Health Claims Understanding Food Labels and Health Claims - Nutrition ... Health Claims & Foods To keep companies from making false claims, the FDA provides food manufacturers' regulations in putting labels on packages that promote health. There are three levels of health claims: A health claim is supported by scientific evidence. An example is "reduces heart disease." Definitions - Health claims on food labels - Food label ... Claims that suggest that the product has the properties of a drug (e.g. the treatment, mitigation or prevention of a disease, disorder or abnormal physical state or its symptoms) or that the product has an effect on the body that is beyond that which is normally associated with a food (e.g. restoring, correcting or modifying organic functions ...
Health claim - Wikipedia Health claims in the United States. In the United States, health claims on nutrition facts labels are regulated by the U.S. Food and Drug Administration (FDA), while advertising is regulated by the Federal Trade Commission. Dietary supplements are regulated as a separate type of consumer item from food or over-the-counter drugs.. Food Objections by consumer advocates Introduction to Food Product Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim". Health Claims on Food Labels | LegalMatch Health claims must be approved by the Food and Drug Administration (FDA) before the manufacturer is allowed to put the claim on one of their food products . There are two ways of obtaining FDA approval: First, the manufacturer can come up with a health claim based on independent scientific studies and evidence. What You Need to Know About Health Claims on Food Labels ... In general, health claims are statements made on food product labels or dietary supplements that boast some type of health benefit. This may seem simple, but the FDA doesn't treat every claim the same way. Label claims come in multiple forms: Health claims (which comprise of authorized health claims and qualified health claims)
Qualified Health Claims | FDA - U.S. Food and Drug ... Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions. The Basics of Food Product Health Claims - LabelCalc For additional information from the FDA on Health Claims, Download this PDF and scroll to Appendix C. Claim #3: Qualified Health Claims. Qualified food product health claims refer to a claim made on a food product label in reference to a disease that does not have to meet the rigorous standards of an Authorized Health Claim. Authorized Health Claims That Meet the Significant ... Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food... Food Packaging Claims - American Heart Association It's important to understand what these claims mean so you can make informed decisions about the food you buy for yourself and your family. There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims.
A Guide to FDA Regulation of Food Labeling Claims ... Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural."
Qualified Health Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim".
FDA needs to make sure 'food labels are ... - Food Dive The paper discusses how the FDA regulates four specific food labeling claims: health claims, qualified health claims, nutrient content claims and structure function claims. Each requires differing ...
FDA sued over health claims on food labels | Center for ... CSPI legal affairs director Bruce Silverglade warns that the FDA's new system will mislead consumers when sketchy health claims begin appearing on food labels. "Food manufacturers could begin claiming that chocolate may reduce the risk of heart disease or that ketchup may reduce the risk of cervical cancer," Silverglade said.
Food Label Claims: What You Can and Can't Trust The FDA is updating its definition for this claim. Until then, companies can make the "healthy" claim if the fats in their foods are mostly mono- and polyunsaturated fats. The healthy claim also...
CFR - Code of Federal Regulations Title 21 - Food and Drug ... (a) Definitions. For purposes of this section, the following definitions apply: (1) Health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements (e.g., a brand name including a term such as "heart"), symbols (e.g., a heart symbol), or vignettes, characterizes the ...
What are some examples of an FDA health claim on a food label? Health Claims - Require premarket approval by the U.S. Food and Drug Administration (FDA) if they are intended for use on the label of foods or dietary supplements. Qualified health claims are based on less scientific evidence than authorized health claims and require disclaimers or qualified wording.
Food labelling and packaging: Nutrition, health claims and ... Food and drink labelling and packaging regulations - what you must show, warnings, health and organic labels and packaging standards. Food labelling and packaging: Nutrition, health claims and ...
Food Labeling | Food and Nutrition Information Center ... The Health Educator's Nutrition Toolkit is designed to help health educators, dietitians, physicians, other health care and nutrition professionals, social workers, youth counselors, and program directors teach consumers about the Nutrition Facts label and how to use the information it provides to make healthier food choices.
FDA perspectives on health claims for food labels FDA perspectives on health claims for food labels Abstract The U.S. Food and Drug Administration's regulatory authority over health claims was clarified in 1990 legislation known as the Nutrition Labeling and Education Act (NLEA).
Enforcement Policy Statement on Food Advertising | Federal ... In addition to requiring nutrition information on virtually all food products, the NLEA directed FDA to standardize and limit the terms permitted on labels, and allows only FDA-approved nutrient content claims and health claims to appear on food labels.7 While the NLEA is designed in part to prevent deceptive and misleading claims on labels ...
Food Politics by Marion Nestle » FDA study: Do added nutrients sell products? (Of course they do)
Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if...
Label Claims for Conventional Foods and Dietary ... there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990...
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
PDF "GMO-Free" Claims and False and Misleading Food Labels—Why ... Through its butterfly label and marketing materials, the Non-GMO Project makes misleading and inaccurate claims, resulting in misbranding. It makes food safety claims that are false and misleading. It's claims interfere with consumers' ability to make wise food purchase decisions.










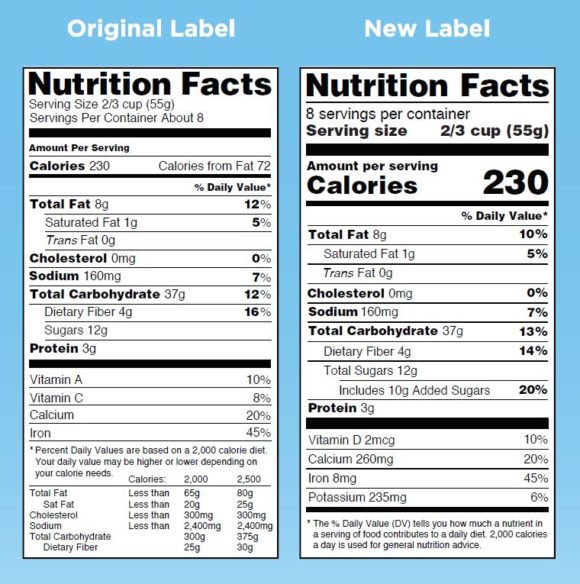
Post a Comment for "43 fda health claims on food labels"